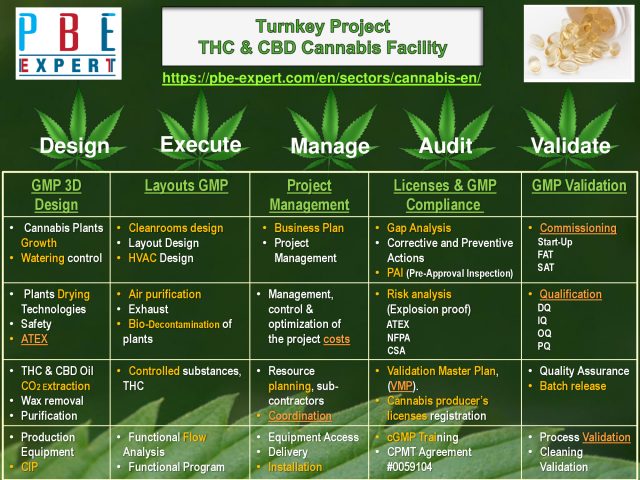
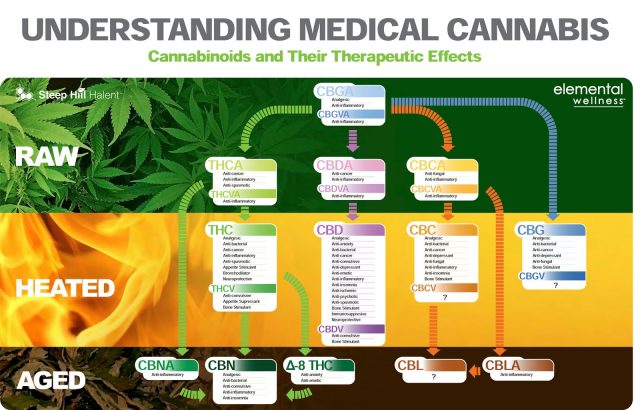
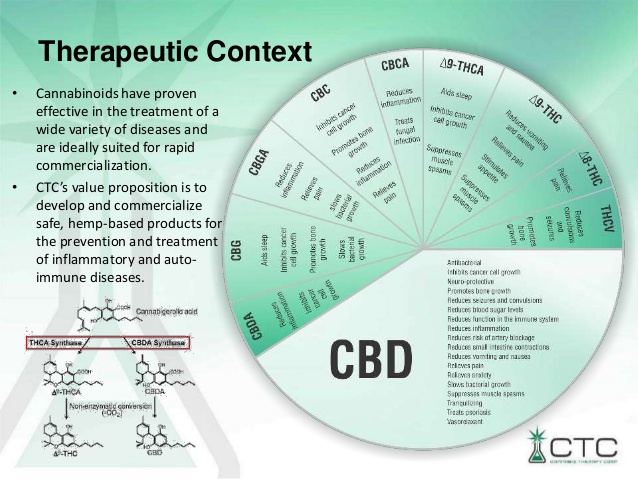
EU-GMP Cannabis
PBE Expert Inc, is a leading consulting Engineering company in EU-GMP Design of Automatic Medical Cannabis Grow and Supercritical CO2 Extraction of THC & CBD Oils ingredients (API, DMF), involving a Health Canada & MAPAQ Expert.
PBE Expert Inc, has successfully accomplished several Medical Cannabis projects, implementing various technologies of Supercritical CO2, Aqueous or Alcoholic Extractions, which require the design and Control of Dehumidification and Environmental conditions that comply with EMA & Health Canada: EU-GMP/ACMPR/ NHP regulatory requirements, ISO-22000 and ATEX standards.

PBE Expert Inc, provides to the cannabis production and processing industry with outstanding EU-GMP expertise in process engineering, extraction technologies of THC & CBD, Layout, Validation, Calibration, Maintenance, Quality Assurance, Compliance and Regulatory Affairs.
PBE Expert Inc offers EU-GMP design services for Medical Cannabis Manufacturing sites: Vertical Growing, Drying, Grinding | Extraction and Purification of active ingredients such as THC and CBD Oils (API, DMF) | Drug Devlopment, Manufacturing Pharmaceutical & Nutraceutical products such as syrup, tablets, soft gel and semi-solid capsules.
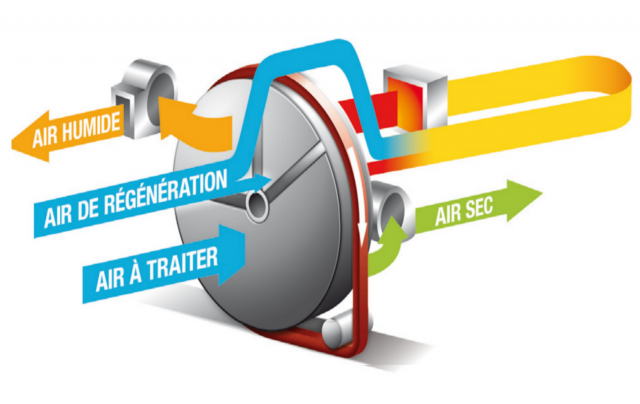
The design of Medical Cannabis Facilities implies the compliance with EU-GMPs and ASME-BPE and ISPE standards, so the design of EU-GMP Layouts is ensured by efficient Environmental Control, Dehumidification, MILDIEW and Odours removal technologies.
- GACP & EU-GMP Cannabis facilities design
- EU-GMP Clean utilities & Pharmaceutical systems
- EU-GMP Sanitary design & Process engineering (ASME-BPE)
- EU-GMP Layouts design & Technical specifications
- EPCMV & Project management
- EU-GMP Compliance Audit, PAI & Training
- EUGMP C&Q – Validation & Maintenance
- EU-GMP Quality assurance & Regulatory affairs
- Licenses Establishment, Cannabis Producers License, NHP registration: NPN.
- “PURS: Product, Process, Plant User Requirements Specifications”, laws and regulations analysis.
- Business plan development, Feasibility story and Project management.
- Design of general “Site Master File: SMF”.
- Transfer technology services.
- Cannabis Plants Drying Technologies, ATEX Safety, energy efficiency.
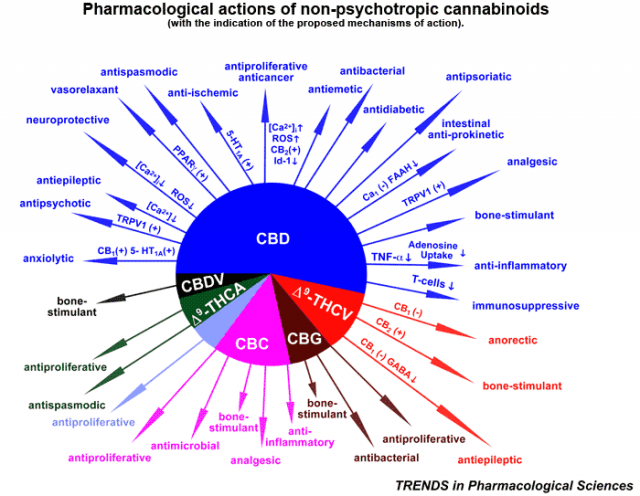
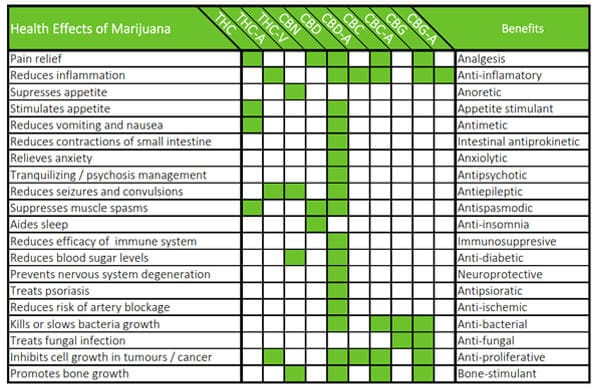
- THC & CBD Cannabis oils extraction and purification technologies.
- QPIC & THC Cannabis Safety classification, Health Canada application for Controlled Substances Licenses.
- Skids of production, processes, Layout, cleanrooms, HVAC, design & specifications.
- Waters quality for plants watering systems & greenhouses HVAC specifications.
- Clean utilities and pharmaceutical systems design & specifications.
- Indoor plants harvest (temperature, humidity, lux light) specifications & HVAC control.
- Personal protective equipment (PPE) and Containment Technologies.
- Personnel, material & finished goods Flows & Cross-Contamination Risks assessment & mitigation.
- Functional Technical Program (PFT) & Facility Design Qualification (FDQ).
- Management of construction projects (EPCMV).
- Audit of Regulatory Compliance and Gap Analysis.
- Validation, Quality Assurance & Regulatory Affairs.
- Warehouse areas, vaults, cold rooms, and Labs quality control areas.
- Design of specific facilities for cannabis.
- Processes, clean utilities and pharmaceutical systems functional analysis and design (ASTM2500).
- Equipment, utilities, systems, production rooms, infrastructure cross matrices.
- Warehouse Layout, cold rooms, and quality control laboratories CQM, specifications.
- Flow functional analysis, Mix-ups & Cross-Contamination Risks mitigation.
- Functional program Technical (PFT).
- Specifications of the Protective Equipment PPE, explosion proof, and containment equipment.
- Cleanrooms integrity, equipment & THC/CBD products quality and purity compliance.
- Risk management, impact & gap analysis and mitigation: RAPA, GAPA, ICH Q9, FMEA, ATEX.
- Warehouse, “Push-Back racking”, cold rooms and quality control Labs design.
- Equipment supplier’s selection and implementation.
- Hazardous chemical & biological waste gas, liquid and solid treatment technologies.
- Support in approval certification “CA“ related to environment protection (MDDELCC).
- Energy and water consumption efficiency optimization. Integration of Renewable Energies “Trigeneration”.
- Sanitary Design & Process Engineering (ASME-BPE) basic and detailed, 3D drawing and performance specifications.
- Development of PURS(9), URS(11), BOD, DDE, PFD, P&ID and “CPP“, & 3D drawings.
- 3D design of Cannabis plants Drying systems, ATEX Safety, energy efficiency.
- 3D design of the THC and CBD cannabis ingredients extraction and purification technologies.
- Specification of materials and internal finishes.
- Sanitary design, dead-legs reduction, optimization of slopes, speeds, CPP.
- Specifications of the valves (diaphragm, Block-Bleed), TC fittings, pumps, filters, exchangers.
- 3D design of processes skids and related equipment.
- 3D design of formulation, mixing and filling tanks.
- 3D design of filling machines, freeze-drying, primary and secondary packaging.
- 3D design of Clean in Place (CIP) skids, COP, sanitisation.
- 3D design of process transfer lines (orbital welding).
- 3D design of the clean utilities, production and distribution: PW, PCA, N2, CO2 …
- 3D design of pharmaceutical systems: HVAC, FFU, CIP, freeze-drying, vacuum, vent, …
- 3D design of protective equipment: laminar flow hood, weighing & sampling (UDAF) cabinet, LEV.
- Interlock between the process equipment and building systems & safety devices.
- Automation, PLC, SCADA, integration, BMS, BAS, ERP, SAP, Industry 4.0.
- Classified areas (A, B, C, D, ISO5-8) and (3D) architectural drawings integration.
- Layout integration of the environmental conditions and the specifications of the products: hygroscopic, electrostatic, explosive, flammable ATEX.
- Development of cleanrooms URS and Critical Environmental Parameters Reviews “CEP“.
- Flow of personnel, equipment, raw material, release of primary & 2nd PGK materials and finished products, waste.
- Functional grouping, pressure & cleanliness cascades and classification of cleanrooms, ATEX, HR%, BSL, OEL.
- Physical (PAL, MAL, corridors) and mechanical segregations, SOP, gowning.
- Functional grouping, pressure & cleanliness cascades and classification of cleanrooms, ATEX, HR%.
- Physical (PAL, MAL, corridors) and mechanical segregations, SOP, gowning.
- Aeraulic diagrams: HVAC, FFU and laminar flow HEPA filters and hoods.
- Weighing & sampling (UDAF) cabinet.
- Design of warehouses, “Push-Back racking”, freezers -20°C, cold rooms 2-8°C.
- Raw materials & drugs, in Quarantine, Stability & Batch release (ERP).
- Vaults of controlled substances, alcohol, cannabis, THC, QPIC Services.
- Explosion proof (XP) design (NFPA, ATEX), and chemical (OEL).
- Layout of quality control of physical & chemical, microbiological and R&D Labs.
- Engineering, validation and construction projects management.
- Suppliers and contractor’s selection.
- Works timeline, resource planning, sub-contractors coordination.
- Management, control & optimization of the project costs.
- Follow-up & technical support, review and approval of the workshop drawings.
- Plans and procedures approval of supervision and health safety (ATEX, NFPA, Safe-brige).
- Equipment accessibility, reception, installation & commissioning.
- Supervision and approval of onsite contractors & suppliers drawings for execution.
- Engineering tests, “Troubleshooting“ & setting of the PLC processes.
- Management and control of work quality and equipment performances.
- Onsite works progress reports follow-up, benchmark and end of the work certificate delivery.
- Update and approval of all the facility, cleanrooms and equipment drawings and documentations “As Built“.
- Certificate of Conformity, release of payments, customer facility and documentation “hand-over”.
- Sites c-GMP compliance certification, chemical containment license, QPIC.
- cGMP compliance Audit and pre-approval inspection (PAI).
- Development of PAI plan and procedure: before, during and after inspection.
- Corrective and Preventive Actions: CAPA, RAPA, GAPA.
- Plans for remediation.
- Management and Implementation of a change control system.
- Continuous Improvement & Quality Audit of supplier’s qualification.
- Drafting and revision of suppliers Quality Assurance Agreements (QAA).
- Development of the Site Master File (SMF).
- cGMP Training: PBE Expert Inc is a CPMT training company with agreement #0059104 & EPIC/OIQ recognition.
- Traceability matrix and URS and validation cross references.
- Validation Master Plan, (VMP).
- Predictive, systemic, and preventive maintenance plan.
- Metrology, calibration program, calibration of the instruments, and traceability.
- Mapping (temperature, humidity) cold room, warehouse, freezer, incubator, autoclave, freeze dryer.
- Commissioning (DQ, FAT, SAT).
- Qualification DQ, IQ, OQ: equipment & classified cleanrooms, quality control Labs equipment, freezer & cold rooms, autoclave, freeze-dryer, oven, stability room, CIP, SIP, HVAC, PW, EPPI, compressed air, N2.
- Sampling Plan, and MACO.
- Process Validation, of cleaning and sterilization (FDA 2011, EMA 2015).
- Sterilization cycles development, Media-Fill tests.
- Computer validation, CSV: BMS, PLC, qualification of ERP software, SAP (GAMP, CFR21-11), data integrity.
- Quality Assurance(22) & batch release.
- QPIC services for controlled substances: THC cannabis, opiates, morphine.
- Drafting of procedures: SOP.
- Manufacturing operation support and health & safety services.
- Change Control, CAPA, Failure Modes Effect Analysis : FMEA.
- Root Causes Analysis “RCA“.
- Annual product review “APR”, Information technology & Data integrity: Capability.
- Risks management, analysis, and mitigation. Gap analysis and impact study RAPA/GAPA FMEA: ICH Q9, ATEX, FMD-135.
- Industry 4.0, IoT, collection and data integrity, CSV, ERP, SAP, LIMS.
- Cannabis producer’s licenses registration, DIN/NPN(6) of THC & CBD.
- Regulatory Affairs, registration of drugs (DIN) and NHP (NPN) and related Vaults.
- Registration of the establishment licenses (DEL) and Medical Devices (MDEL).
Regulations
EU-GMP| FDA | EMA| Health Canada | GCC | ISO14644 | ASME-BPE | ISPE | ICH Q9 | FMEA| ATEX | NFPA | HSE | SIMDUT